
Methanol - Thermophysical Properties - Chemical, physical and thermal properties of methanol, CH 3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol).Ethylene - Thermophysical Properties - Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas.Temperature - Density of Ethyl Alcohol aqueous solutions. Ethanol Water Mixtures - Densities vs.Temperature and Pressure - Online calculators, figures and tables showing specific heat, Cp and Cv, of gasous and liquid ethanol at temperatures ranging from -25 to 325 ☌ (-10 to 620 ☏) at atmospheric and higher pressure - Imperial and SI Units. Temperature and Pressure - Online calculator, figures and tables showing density and specific weight of ethanol at temperatures ranging from -25 to 325 ☌ (-10 to 620 ☏) at atmospheric and higher pressure - Imperial and SI Units. Ethanol - Density and Specific Weight vs.Temperature and Pressure - Online calculator, figures and tables showing dynamic and kinematic viscosity of ethanol, C 2H 5OH, at varying temperature and pressure - Imperial and SI Units. Ethanol - Dynamic and Kinematic Viscosity vs.Benzene - Thermophysical properties - Chemical, physical and thermal properties of benzene, also called benzol.Acetone - Thermophysical Properties - Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid.Viscosities - Viscosities of products and chemical species at varying conditions.
Melting and Freezing Points - Melting and freezing points of elements and chemical species at varying conditions.Boiling Points - Boiling points of elements, products and chemical species at varying conditions.Densities - Densities of solids, liquids and gases.Thermodynamics - Work, heat and energy systems.Material Properties - Material properties of gases, fluids and solids - densities, specific heats, viscosities and more.Involving velocity, pressure, density and temperature as functions of space and time. Fluid Mechanics - The study of fluids - liquids and gases.The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. It also shows the saturation pressure with changes in temperature.Īt the critical point there is no change of state when pressure is increased or if heat is added. The curve between the critical point and the triple point shows the ethanol boiling point with changes in pressure. The phase diagram for ethanol shows the phase behavior with changes in temperature and pressure. However, at low temperature and/or very high pressures it becomes a solid. See also more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,Īs well as Thermophysical properties of: Acetone, Acetylene, Air, Ammonia, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethylene, Helium, Hydrogen, Hydrogen sulfide, Methane, Methanol, Nitrogen, Oxygen, Pentane, Propane, Toluene, Water and Heavy water, D 2O.Įthanol is a liquid at standard conditions. Specific Heat (Heat Capacity), C p and C v.Specific Gravity (liquid) (relativ to water)įollow the links below to get values for the listed properties of ethanol at varying pressure and temperature:

Log K OW (Octanol/Water Partition Coefficient) Specific heat capacity, Cv (isochoric) (gas) Specific heat capacity, Cp (isobaric) (gas)
Density of ethanol full#
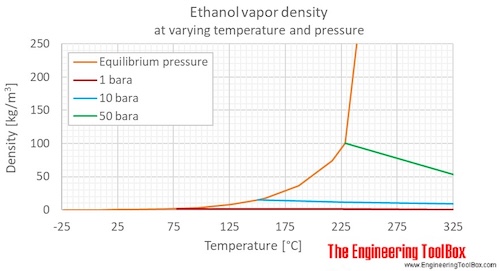
Values are given for liquid at 25 oC /77 oF / 298 K and 1 bara, if not other phase, temperature or pressure given.įor full table with Imperial Units - rotate the screen! Property The phase diagram of ethanol is shown below the table.Ĭhemical, physical and thermal properties of ethanol : Ethanol is also used as a clean-burning fuel source. The compound is widely used as a chemical solvent, either for scientific chemical testing or in synthesis of other organic compounds. It also has medical applications as an antiseptic and disinfectant. It is a psychoactive substance and is the principal type of alcohol found in alcoholic drinks. It is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts.Įthanol is most commonly consumed as a popular recreational drug. Ethanol (Ethyl Alcohol), C 2H 5OH, is a volatile, flammable, colorless liquid with a slight characteristic odor.


 0 kommentar(er)
0 kommentar(er)
